Background:
EP0042 was developed as an orally bioavailable potent inhibitor of FLT3 and Aurora kinases. Aurora kinases play an important role in acute myeloid leukemia (AML) and have been implicated in the development of resistance to FLT3 inhibitors (Joshi S et al. Cancer Cell 2021;39:999-1014, 8). Dual inhibition of FLT3 and Aurora kinase has been shown to overcome acquired resistance to selective FLT3 inhibition both in vitro and in vivo (Moore A et al. Leukemia 2012;26:1462-70; Tariq M et al. Br J Cancer 2021;125:966-74). Preliminary data from this ongoing Phase 1 study of EP0042 in relapsed/refractory AML were presented previously (Taussig et al. Blood 2022:140(Supplement 1): 6222-6223). Here we present further data from the dose finding and dose optimisation cohorts from the study.
Methods
The primary objective of this phase I/IIa, multi-centre study is to investigate safety/tolerability and to establish both the MTD and optimal dose of EP0042 when used as monotherapy, or in combination with established standard treatments in patients with AML, myelodysplastic syndrome (MDS) or chronic myelomonocytic leukemia (CMML) who have relapsed on or are refractory to previous therapy. Secondary objectives include PK characterisation and assessment of anti-tumour activity.
Results
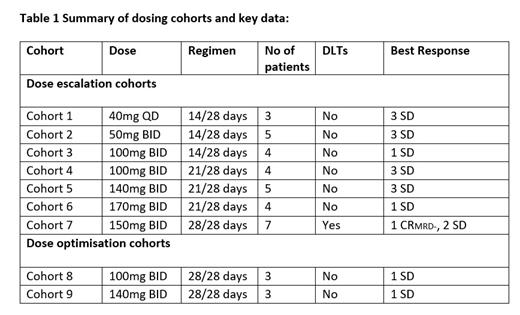
As of July 13 th 2023, 38 patients (17 f/21 m) were enrolled across 9 different cohorts. Median age 72 years, range 25-84. Patients presented with relapsed/refractory AML (34), CMML (1) or MDS (3). 9 patients had FLT3 mutated AML at the point of entry (All FLT3 ITD). Patients were administered doses ranging from 40mg to 170mg, QD or BID, with different schedules, across 7 cohorts (Table 1). One Cycle = 28 days. 150mg BID 28/28 days was identified as the MTD with 2 DLTs identified, Grade 3 somnolence and ataxia, and Grade 3 encephalopathy. Two dose optimisation cohorts (Table 1) were subsequently initiated to further optimise the potential RP2D. These cohorts are currently ongoing.
The median number of prior treatments was 2 (range 1-6). 12 patients received a prior FLT3 inhibitor including midostaurin, gilteritinib or sorafenib and 8/12 patients received ≥2 prior FLT3 inhibitors. 3 patients received prior ASCT. The most common treatment-emergent adverse events (TEAEs) ≥20% were: diarrhea (54.1%), fatigue (54.1%), febrile neutropenia (51.4%), decreased appetite (43.2%), dyspnea (35.1%), somnolence (29.7%), constipation (27.0%), ataxia (27.0%) and nausea (24.3%). 23.3% of TEAEs were > grade 3. The most common treatment related AEs (TRAEs, all grades ≥10%) were decreased appetite (29.7%), somnolence (29.7%), ataxia (27.0%), fatigue (24.3%), diarrhea (13.5%), nausea (13.5%) and dizziness (16.2%). No treatment-related death has been reported across all cohorts. Most events were manageable by temporary dose reductions and or/short interruptions. All events of ataxia and somnolence were reversible after a short treatment interruption.
Across all cohorts, 18 patients achieved stable disease (SD) as best response (median no. of cycles = 3, range 1-11). 1 patient continued treatment for 11 cycles (100mg BID, FLT3wt) and another for 8 cycles (140 mg BID, FLT3-ITD) with clinical benefit. 1 patient achieved a best response of CR MRD- (150mg BID, FLT3wt) with aCRi first detected at C6D1 (2% blast, down from 38% at screening and 80% at C3D1) and confirmed with CR MRD- at C7D1 by flow cytometry. Treatment is still ongoing at C10 and patient remains transfusion independent for 4 months. Evidence of peripheral blast response was observed in 5 patients, with complete disappearance of peripheral blasts compared to baseline after a median time of 2 months on treatment. Data from the ongoing dose optimisation cohorts will be presented at the congress.
Conclusions
This extended data set confirms thatEP0042 has acceptable tolerability and safety. Two patient cohorts are ongoing to establish the optimum continuous dose regimen to take forward. Promising evidence of clinical benefit has been observed including a durable CR MRD-. This remission in a patient with FLT3 wild type AML indicates that EP0042 may have broader activity in AML, beyond FLT3 mutated disease.
Dose optimisation cohorts are continuing to identify the RP2D. Further cohorts are planned for evaluation of EP0042 in combination with standard of care therapies.
Disclosures
Taussig:Ellipses Pharma: Consultancy. de Leeuw:Takeda: Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy; Roche: Consultancy; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Ellipses Pharma: Research Funding. Jongen-Lavrencic:Ellipses Pharma: Research Funding. O'Nions:Ellipses Pharma: Research Funding; Jazz: Honoraria; Astellas: Honoraria; Abbvie: Consultancy. Koko:Ellipses Pharma: Research Funding. Searle:Shattuck Labs: Consultancy; Sanofi: Consultancy; Abbvie: Honoraria, Other: Conference travel; Janssen: Honoraria, Other: Conference travel. Arkenau:Ellipses Pharma: Current Employment. Brook:Ellipses Pharma: Current Employment. Burmester:Ellipses Pharma: Current Employment. Fisher:Ellipses Pharma: Current Employment. Stoddart:Ellipses Pharma: Current Employment. Tan:Janssen: Research Funding; Abbvie: Research Funding; Beigene: Research Funding; Ellipses Pharma: Research Funding; Novartis: Research Funding; Gilead: Research Funding; Loxo Oncology at Lilly: Research Funding; Kartos Therapeutics: Research Funding; Shanghai EpimAb Biotherapeutics: Research Funding; Qilu Pharmaceuticals Co. Ltd: Research Funding; Genor Biopharma Co. Ltd: Research Funding; Constellation Pharmaceuticals, Inc: Research Funding; Protagonist Therapeutics, Inc: Research Funding; Ichnos Sciences SA: Research Funding; Bristol-Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal